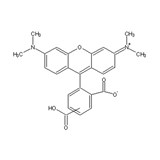
5(6)-TAMRA [5-(and-6)-Carboxytetramethylrhodamine] "Formulated"
- Cat.Number : AS-81120
- Manufacturer Ref. :
-
Availability :
In stock
- Shipping conditions : Ice fees will apply
Alternative choices
5(6)-TAMRA is the mixture of two carboxy tetramethylrhodamine (TMR) isomers. It is used to modify amino and hydroxy groups using EDC-mediated couplings when there are difficulties in using 5(6)-TAMRA, SE. TAMRA is one of the most popular fluorophores used in various bioconjugations. The dye is specially formulated to easily dissolve in DMF and to convert to the NHS ester.
Specifications
| Chemistry | |
| CAS registry number |
|
|---|---|
| Molecular Formula |
|
| Molecular Mass/ Weight |
|
| Properties | |
| Absorbance (nm) |
|
| Emission (nm) |
|
| Color | |
| Quantity & Purity | |
| Purity |
|
| Storage & stability | |
| Form |
|
| Resuspension condition |
|
| Storage Conditions |
|
| Activity | |
| Application | |
| Detection Method | |
| Research Area | |
| Sub-category Research Area | |
| Usage |
|
Downloads
You may also be interested in the following product(s)

Citations
Monitoring 14-3-3 Protein Interactions with a Homogeneous Fluorescence Polarization Assay.
J Biomol Screen . 2006 Apr 01 ; 11(3) 269 | DOI : 10.1177/1087057105284862
- Y. Du
References
A novel flow cytometric method for quantifying phagocytosis of apoptotic cells
Cytometry . 1997 Feb 01 ; 27(2) 145 | DOI : https://doi.org/10.1002/(SICI)1097-0320(19970201)27:2<145::AID-CYTO6>3.0.CO;2-F
- KL. Hess
- et al
Visualizing differences in ligand-induced β-arrestin–GFP interactions and trafficking between three recently characterized G protein-coupled receptors
J Neurochem . 2001 Dec 20 ; 77(2) 476 | DOI : https://doi.org/10.1046/j.1471-4159.2001.00269.x
- NA. Evans
- et al