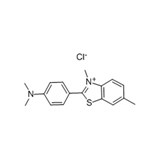
Thioflavin T UltraPure Grade - 1 g
- Cat.Number : AS-88306
- Manufacturer Ref. :
-
Availability :
In production
The benzothiazole dye thioflavin T (ThT) is a classic amyloid stain for senile plaques containing βA4 peptide in Alzheimer's disease brain. ThT also binds rapidly and specifically to the anti-parallel β-sheet fibrils formed from synthetic β-amyloid (1-42), but does not bind to monomer or oligomeric intermediates. The fibrillar β-sheet-bound dye species undergoes a characteristic 120 nm red shift of its excitation spectrum that may be selectively excited at 450 nm, resulting in a fluorescence signal at 482 nm. ThT is a useful probe for the aggregated fibrillar state of β-amyloid (1-42) fibrils as the amyloid-specific fluorescence reports only fibrillar species. The binding of ThT does not interfere with the aggregation of this peptide into amyloid fibrils. The putative conformational changes detected by the ThT fluorescence suggest that small pharmacologic ligands can perturb and possibly dissociate Aβ amyloid fibrils.
Specifications
| Chemistry | |
| CAS registry number |
|
|---|---|
| Molecular Formula |
|
| Molecular Mass/ Weight |
|
| Properties | |
| Absorbance (nm) |
|
| Emission (nm) |
|
| Color | |
| Quantity & Purity | |
| Purity |
|
| Storage & stability | |
| Form |
|
| Resuspension condition |
|
| Storage Conditions |
|
| Activity | |
| Application | |
| Biomarker Target | |
| Detection Method | |
| Research Area | |
| Sub-category Research Area | |
| Usage |
|
Downloads
You may also be interested in the following product(s)

SensoLyte® Thioflavin T ß-Amyloid (1-40) Aggregation Kit - 1 kit

SensoLyte® Thioflavin T ß-Amyloid (1-42) Aggregation Kit - 1 kit
Citations
Heterogeneous amylin fibril growth mechanisms imaged by total internal reflection fluorescence microscopy.
Biochemistry. . 2011 Mar 21 ; 50(14) 2808 | DOI : 10.1021/bi101908m
- SM. Patil
Spectral properties and factors determining high quantum yield of thioflavin T incorporated in amyloid fibrils.
Spectroscopy . . 2009 Aug 28 ; 24 4 | DOI : 10.3233/SPE-2010-0418
- A.I. Sulatskaya
Charge transfer process determines ultrafast excited state deactivation of thioflavin T in low-viscosity solvents.
J Phys Chem . 2010 Aug 19 ; 114(32) 8345 | DOI : 10.1021/jp105186z
- V. Stsiapura
In vitro formation of amyloid from α-synuclein is dominated by reactions at hydrophobic interfaces.
J Am Chem Soc . 2010 Jul 21 ; 132(28) 9797 | DOI : 10.1021/ja102896h.
- J. Pronchik
References
Direct observation of amyloid fibril growth monitored by thioflavin T fluorescence
J Biol Chem . 2003 May 09 ; 278(19 16462 | DOI : 10.1074/jbc.C300049200
- T. Ban
- et al
IMPY: an improved thioflavin-T derivative for in vivo labeling of beta-amyloid plaques
Brain Res . 2002 Nov 29 ; 956(2) 202 | DOI : https://doi.org/10.1016/S0006-8993(02)03436-4
- MP. Kung
- et al
Thioflavin T Is a Fluorescent Probe of the Acetylcholinesterase Peripheral Site That Reveals Conformational Interactions between the Peripheral and Acylation Sites
J Biol Chem . 2001 Jun 29 ; 276(26) 23282 | DOI : https://doi.org/10.1074/jbc.M009596200
- GV. De Ferrari
- et al