Frequently asked questions
Products & Services
Assay Kits
Amyloid processing or degradation: which protease activities can be detected using SensoLyte® assay kits?
We offer several SensoLyte® assay kits that can detect proteases involved in amyloid precursor protein (APP) or beta-amyloid, processing or degradation.
| Target | Assay Kit Name | Catalog Number |
| ADAM10 | SensoLyte® 520 ADAM10 Activity Assay Kit Fluorimetric | AS-72226 |
| BACE-1 | SensoLyte® 520 ß-Secretase (BACE1) Activity Assay Kit Fluorimetric | AS-71144 |
| BACE-2 |
SensoLyte® 520 BACE2 Activity Assay Kit Fluorimetric |
AS-72225 |
| Cathepsins B | SensoLyte® 520 Cathepsin B Assay Kit Fluorimetric | AS-72164 |
| Cathepsins D |
SensoLyte® 520 Cathepsin D Assay Kit Fluorimetric |
AS-72170 |
| ECEs |
SensoLyte® 520 ECEs Activity Assay Kit |
AS-72243 |
| IDE | SensoLyte® 520 IDE Activity Assay Kit | AS-72231 |
| Neprilysin | SensoLyte® 520 Neprilysin Activity Assay Kit | AS-72223 |
| MMP-2 |
SensoLyte® Plus 520 MMP-2 Assay Kit Fluorimetric and Enhanced Selectivity |
AS-72224 |
| MMP-9 |
SensoLyte® Plus 520 MMP-9 Assay Kit Fluorimetric and Enhanced Selectivity |
AS-72017 |
| TACE / ADAM17 / a-Secretase |
SensoLyte® 520 TACE (a-Secretase) Activity Assay Kit Fluorimetric |
AS-72085 |
Aggregation of beta-amyloid 1-40 and 1-42: does AnaSpec offer a method for its evaluation?
Yes. AnaSpec developed SensoLyte® ThT Aggregation kits to test for amyloid aggregation, of beta-amyloid 1-42 or beta-amyloid 1-40. These kits include all the reagents needed to perform the test, including a step by step protocol.
| Product Name | Catalog Number |
| SensoLyte® Thioflavin T ß-Amyloid (1-40) Aggregation Kit | AS-72213 |
| SensoLyte® Thioflavin T ß-Amyloid (1-42) Aggregation Kit | AS-72214 |
|
Figure 1. An increase of fluorescence signal is correlated with an increase of Aβ42 fibril formation. Phenol Red, o-Vanillin, Tannic Acid, Morin, and Dopamine, were added at 100μM final concentration to inhibit Aβ42 aggregation. Fluorescence signal was monitored at Ex/Em= 440/484 nm every 5 minutes at 37 ºC with 15 seconds shaking between reads (Flexstation II-384 fluorimeter, Molecular Devices). |
What is the difference between the SensoLyte® 490 and 520 series of protease activity assay kits?
Both the 490 and 520 series of protease activity assay kits offer specific benefits depending on the goals of the analysis.
The SensoLyte® 520 kits provide higher sensitivities over the 490 SensoLyte® kits. This is attributed to the dyes that are used in the substrates of that kit series. The 520 substrates, utilize a dye with a longer excitation (Ex) and emission (Em) wavelength. At these longer wavelengths, the interference from cellular components and test compounds, is at a minimum.
Although less sensitive, the SensoLyte® 490 series of kits can perform a larger number of assays per kit, at 500 assays in a 96-well format, while the 520 series kits can perform 100 assays in a 96-well format.
Do you sell separate components from your SensoLyte® assay kits?
In bulk quantities of 15 units or more, we do sell separate assay kit components for most kits. However, certain kits including ELISA kits, are exempt.
What kind of microplates should be used with SensoLyte® fluorimetric kits?
For bottom reading fluorimetric plate readers, black plates with clear bottom should be used.
For top reading fluorimetric plate readers, opaque plates (black or white) are recommended, but black plates with clear bottom can also be used.
Can RIPA buffer be used for preparation of test samples to be analyzed using SensoLyte® Assay Kits?
It depends on the composition of RIPA buffer. For example, SDS and Na Deoxycholate are denaturing anionic detergents and should not be used as they can damage the enzyme and it’s activity. Protease inhibitors added to the RIPA buffer should not be specific for the target protease.
Can SensoLyte® protease activity kits can be used for different species?
Although we validate most of our substrates with human enzymes, it may be possible that they are cleaved by enzymes from other species as well.
Example: MMP-9 substrate from Cat#AS-71155 SensoLyte® 520 MMP-9 Assay Kit is cleaved by human and mouse MMP-9 enzyme.
Labeling and Dyes
What is the recommended protein concentration when using an AnaTag labeling kit?
A concentration of ≥ 2mg/mL is recommended.
In which buffer can my protein be dissolved in when using AnaTag™ kits?
To ensure optimal labeling efficiency, avoid buffer additives such as reducing agents (e.g., DTT), protein stabilizers (e.g., BSA), and sodium azide, as they may interfere with the reaction chemistry.
In addition, buffers containing primary amines, including Tris, glycine, and ammonium salts should be avoided, as they can compete with target molecules during labeling.
Please refer to the product data sheet for recommended buffer and compatible conditions.
How can free dyes be removed from my labeled protein?
The free dyes are removed during the purification step via the spin column that is provided with the kit.
What purification yields can I expect?
On average, we observe 70-80% yields.
After labeling my protein, can I use a dialysis membrane to remove excess dye?
Yes, but it is important to use a membrane molecular weight cut off that is small enough to prevent protein from being lost through the membrane pores.
Where does the binding occur between the dye and my protein?
Dyes in AnaTag kits have a succinimidyl ester reactive group which can bind to any free amine available on your protein. Hence the dye can bind to any Lysine residues and protein N-terminus.
Peptides
How should I store my peptides?
Lyophilized peptides
For the long-term storage of peptides, we recommend that peptides be stored as a solid powder. Lyophilized peptides can be stored at temperatures of -20 °C or lower with little degradation. AnaSpec’s catalog peptides are checked every 2 years from the date of QC release to ensure that these peptides are within the required purity specification. AnaSpec recommends that customers also re-check their custom peptides every 2 years from the date of QC release.
Peptides in solution
Peptides in solution are much less stable and are susceptible to degradation. Solutions of water used to dissolve peptides should be sterile and purified.
In solution, some amount of the peptide may degrade depending on the amino acids present in the sequence. A few examples of which are shown below:
- Peptides containing methionine, cysteine, or tryptophan residues should have limited storage time in solution due to oxidation. These peptides should be dissolved in oxygen-free solvents.
- Glutamine and asparagine can deamidate to Glu and Asp, respectively.
- Cysteines can undergo oxidative cyclization to form Cys-Cys disulfide bridges (intra or inter disulfide bridges can form).
- Charged residues (Asp, Glu, Lys, Arg, His) are hygroscopic (take up water from moisture in the air) and will easily form a viscous clear oil. This physical change may not affect the properties of the peptide. It is recommended that such peptides be first lyophilized to remove trace amounts of water, degassed with N2 to ensure that there is no water vapor in the vial, and quickly capped.
To prevent any damage caused by repeated freeze-thaw cycles, we recommend that the user only dissolves the amount of peptide needed for the immediate experiment. Any excess solutions should be stored at ≥ -20 oC until needed.
Avoid moisture
As moisture will greatly reduce the long-term stability of peptides, we recommend that the peptide should be allowed to equilibrate to room temperature in a desiccator before opening the vial. Once the peptide has been dispensed, any remaining peptide in the tube should be gently purged with anhydrous nitrogen, the container recapped, sealed with parafilm, and stored at -20 °C.
How do I solubilize my peptides?
Peptide solubility characteristics vary strongly from one peptide to another. Residues such as Ala, Cys, Ile, Leu, Met, Phe, and Val will increase the chance of the peptide being hydrophobic and dissolving in aqueous solutions. AnaSpec catalog peptides are tested for their solubility in certain solvents. This information is located on every QC Datasheet that is shipped along with the peptide. AnaSpec recommends that customers adhere to the below guideline for their custom peptides.
Solubility properties
Peptide solubility is highly dependent on the amino acid sequence. Hydrophobic peptides (high propensity of A, F, G, V, L, I, M, W, P) in nature, will require an organic solvent to dissolve. Acidic peptides (high propensity of D, E in the peptide sequence) require a basic aqueous buffer to dissolve, while basic peptides (high propensity of K, H, and R) require an acidic aqueous buffer to dissolve.
Selection of solvent
Taking into consideration the limitations of your assay, we recommend that the following guideline be used to determine the best solvent to dissolve your peptide
Hydrophobic peptides
To reconstitute a hydrophobic peptide, add 100 µL of DMSO and sonicate until a homogenous solution forms. Next, add your buffer of choice to form a 1 mg/mL solution (a higher concentration of peptide will require a greater amount of DMSO).
Hydrophilic (acidic) peptide
To reconstitute an acidic peptide, add 100 µL of 1% NH4OH to 1 mg of the peptide and vortex. After the formation of a clear solution, add your buffer of choice to form a 1 mg / mL solution.
Hydrophilic (basic) peptide
To reconstitute a basic peptide, add distilled water to the 1 mg of peptide and vortex.
Use and storage
Reconstituted peptides can be stored frozen at -20°C for a short time, but it is advisable to prepare multiple aliquots to avoid multiple freeze-thaw cycles. We recommend that all aliquoted solutions be lyophilized if the peptide is going to be stored for extended periods at -20 oC.
Peptides with a propensity to aggregate
For peptides that tend to aggregate due to the presence of multiple Cysteines, we recommend that these peptides be dissolved in degassed solutions and/or acidic conditions.
Additionally, some types of peptides have a propensity to form secondary and tertiary structures once dissolved. We recommend that these peptides be first dissolved in solvents such as HFIP (hexafluoroisopropanol) and then evaporated using a stream of nitrogen. The HFIP helps to break up the hydrogen bonding network that aids in forming the secondary and tertiary structures.
What is the difference between net vs gross peptide weight?
The industry standard is to deliver peptides in a lyophilized form as a Gross weight. Gross peptide weight is the total weight of all components present in the lyophilized powder. This includes the peptide of interest, any peptide impurities, water, residual solvents, and counterion.
On the contrary Net peptide weight, calculated from the Net peptide content, is the weight of only the peptide component present in the lyophilized powder. This offers more accurate concentrations of your subsequent peptide solutions. Hence, we recommended net peptide weight amounts for Custom Peptide orders.
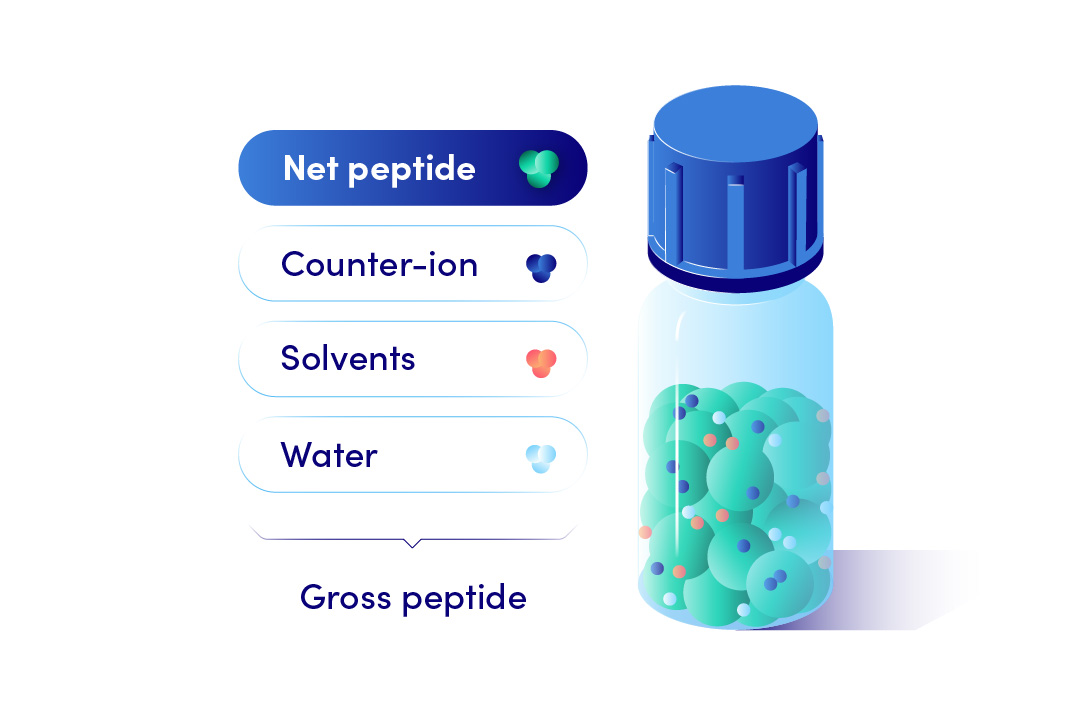
Net Peptide Content measurement is performed by amino acid analysis (AAA; limited accuracy but requires a low material amount) or elemental analysis (CHN; requires milligrams of peptide but is more accurate). Both methods yield a percentage value (ie., 75% net peptide content).
The Net Peptide Content depends on the amount of counterion present*, which is dependent on the peptide sequence. Essentially, the higher the peptide charge, the lower the peptide content (read more at FAQ: What is a Peptide Counterion?).
*Note: The element used to calculate net peptide content is based on the peptide counter-ion. This is done to ensure that the counter-ion does not contribute to the peptide content amount. For example, when TFA or acetate are the counter-ion, nitrogen is used because it is found in the peptide but not in TFA or acetate. Carbon or Nitrogen can be used used when chloride is the counter-ion for the same reason.
Net Peptide Weight calculations:
Net Peptide Quantity (total peptide) — method we use for net custom peptide orders.
Multiply the Net Peptide Content Percentage (decimal form) by the Peptide Gross Weight, to obtain the amount of Net Peptide Quantity (total peptide).
For example:
For 5 mg gross peptide/vial:
Peptide net content is 84%.
Exact amount of total peptide = 0.84*5 mg=4.2 mg total peptide
Net Peptide of Interest Quantity—most accurate — customer can use this method on their own to calculate peptide of interest.
Multiply Net Peptide Content Percentage by the Peptide Gross Weight, and the Peptide Purity (by HPLC), to calculate the exact amount of the peptide of interest you have. The net peptide percent (decimal form) is multiplied by peptide purity (decimal form).
For example:
For 5 mg gross peptide/vial with:
Peptide net content is 84%.
Exact amount of peptide of interest is = 0.84*0.96*5 mg=4.03 mg
What type of chemistry do you use?
AnaSpec utilizes solid-phase Fmoc chemistry rather than BOC chemistry. Fmoc chemistry allows for the removal of protecting groups using mild conditions during peptide elongation, while BOC chemistry requires the use of a strong acid to remove the protecting groups during peptide elongation. Additionally, Fmoc chemistry utilizes trifluoroacetic acid (TFA) to remove the peptide from the resin, while BOC chemistry utilizes hydrofluoric acid (HF). HF is colorless, highly corrosive, and a contact poison.
Therefore, AnaSpec employs Fmoc chemistry as it is more mild, flexible, and versatile compared with BOC chemistry.
What quality control (QC) testing does AnaSpec perform on research grade peptides?
| QC Testing Attributes | Custom Research Grade |
Catalog Research Grade |
|---|---|---|
| Appearance | ✔ | ✔ |
| Purity by % peak area by analytical HPLC | ✔ | ✔ |
| Identity by Mass Spec analysis | ✔ | ✔ |
| Solubility Testing | optional | ✔ |
| Peptide sequence confirmation | optional | optional |
| Counterion content | optional | optional |
| Metal content | optional | optional |
| Endotoxin | optional | optional |
| Bioburden | optional | optional |
Checkmark indicates standard testing.
Optional add-on testing services available upon request.
GMP QC testing is individualized per request.
QC datasheet is shipped with each peptide that includes:
- Testing attributes
- Test method
- Acceptance criteria
- QC results
Raw data for each test can be provided upon request.
How do I calculate molarity of my peptide?
Molarity refers to the molar concentration of a solution, which is the number of moles of solute dissolved in 1 liter of solution, expressed as mol/L, or M.
Molarity [M] = Mass / (Volume x Molar Mass); Mole = Concentration (g/L) x Volume (L)/MW (g/mol)
Example:
Given: 1mg of dry peptide powder with MW: 20KDa (Molar mass of peptide is 20 g/mol)
To determine molarity with known mass and known volume
For a 1ml solution of this peptide:
Molarity = Mass (0.001g) / (volume (0.001L) x Molar Mass (MW 20,000) = 50μM
To determine mass to achieve a certain molarity:
If for your assay, you need 0.01mM working peptide solution in 1ml of water, then calculate mass required as follows:
Mass = Molarity x Volume x Molar Mass
Mass = 0.01mM x (1/1000 L) x 20,000g/mol = 0.0002g
Hence, you will need 0.2mg/ml of peptide to have a working solution of 0.01mM.
For accurate molarity calculations, it’s important to understand which peptide weight to use.
Read more at FAQ: What is the difference between net vs gross peptide weight?.
How are the peptides supplied?
Peptides are shipped as lyophilized powder or oil at room temperature via an express courier. On request, we can also deliver your peptides in solution.
How do I know if my peptide will be soluble?
The solubility of a peptide in a given solvent is dependent on the amino acid sequence and often hard to predict. In the case of catalog peptides, the solubility information is listed on the QC datasheet. For custom peptides, please see the section entitled "How should I solubilize my peptide".
What spacer is there in a biotinylated peptide?
The standard biotin modification does not include a spacer. Spacers (PEG, X, etc.) are available on request. Please contact service@anaspec.com for more guidance on the type of spacers that we offer.
How do you confirm if a peptide is cyclized?
Generally, AnaSpec can perform seven types of cyclization reactions as shown below:
Head-to-tail, side-chain-to-head, side-chain-to-tail, and side-chain-to-side-chain cyclization products are usually confirmed by both a shift in molecular mass of 18 mass units in the mass spectrum as well as a change in the retention time in the analytical HPLC spectrum.
A disulfide bridge cyclization is typically confirmed by a mass shift of 2 mass units in the mass spectrum and a shift in the retention time of the analytical HPLC spectrum. Ellman’s testing may also be used to confirm absence of free thiol groups.
A hydrocarbon-stapled peptide consists of alkene that has been formed using a ruthenium catalyst. The hydrocarbon-staple ensures that the peptide conforms to an α-helix motif, which has been shown to be useful for some therapeutic applications. This staple is confirmed by both a shift in the molecular mass (loss of 28 mass units) in the mass spectrum as well as a change in the retention time in the analytical HPLC spectrum.
Thioether-bond (-CH2-S-CH2-) containing peptides are another type of cyclic peptide. This type of cyclization is confirmed by both a shift in the molecular mass in the mass spectrum as well as a change in the retention time in the analytical HPLC spectrum.
Do you synthesise 'wobble' peptides?
AnaSpec can synthesize ‘wobble’ or degenerate peptides. As there are known differences in the incorporation rates of different amino acids, we can compensate for these rates to produce roughly equimolar amounts of each ‘wobble’ peptide.
Do you synthesize isotopically-labeled peptides?
AnaSpec can synthesize peptides containing non-radioactive atoms from commercially available building blocks. This includes amino acids containing 13C and/or 15N for Nuclear Magnetic Resonance (NMR) or Mass Spectrometry applications, just to name a few.
What is the difference between peptide content and peptide purity?
Peptide content is not an indication of the peptide purity as these are two separate measurements. Purity is determined by analytical HPLC and indicates the presence/absence of other peptidic contaminants (i.e. truncated peptides).
Net peptide content determines the actual amount of the peptide (peptide of interest as well as the peptide contaminants) present in the sample. The net peptide content is traditionally measured by Amino acid analysis (AAA; limited accuracy but requires a low amount of material) or elemental analysis (CHN; requires milligrams of peptide but is more accurate).
How do you generally purify your peptides?
AnaSpec’s peptides are generally purified by reverse-phase preparative HPLC, using 2 buffers containing 0.1% trifluoroacetic acid (TFA). The first buffer (Buffer A) is composed of 0.1 % TFA in deionized water and the second buffer (Buffer B) is composed of 0.1 % TFA in acetonitrile (ACN).
Generally, crude peptides are first dissolved in a solution of either Buffer A, some amount of Buffer B, or an organic polar solvent such as DMSO. If an organic polar solvent or Buffer B was used, this clear solution will first be diluted with Buffer A. Next, the peptidic solution is filtered and loaded onto the preparative HPLC system. Utilizing an optimized method gradient, fractions are then collected based on the absorbance readings. Each fraction is then analyzed via an analytical HPLC to ensure that the purity of each fraction meets the required purity specification.
These “pure” fractions are then pooled together and lyophilized. The “pure” powder is then tested for both purity and identity to ensure that the correct peptide has been manufactured.
Finally, an independent QC group at AnaSpec again tests the peptide to ensure that all of the testing attributes meet the required specifications.
What is Mass Spectrometry and why do we use it?
Mass spectrometry (MS) is an analytical tool used to determine the identity of molecules. In a typical MS procedure, a sample is ionized by bombarding the sample with a beam of electrons. The results are a plot of intensity as a function of the mass-to-charge ratio (m/z). These measurements can often be used to calculate the exact molecular weight of the sample components as well.
MALDI-TOF (Matrix-Assisted Laser Desorption Ionization - Time of Flight) Mass Spectrometry
MALDI-TOF is a technique that uses a pulsed laser that generates a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. MALDI-TOF instruments are often equipped with a reflectron that functions to increase the time of flight between ions of different m/z, which yields an increase in resolution.
ESI (Electrospray Ionization) Mass Spectrometry
ESI a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized.
Can you explain the M+Na and M+K mass peaks in MALDI spectra?
It is common to see Na (Sodium) and K (Potassium) adducts of the peptide in the mass spectrum. Sodium and Potassium analytes originate from the water used to purify the peptide. Even distilled and deionized water has trace amounts of sodium and potassium ions, which can never be entirely removed. These analytes associate with the free carboxyl groups of the peptide and become easily ionized during the ionization step.
As a result, the mass spectrum of some peptides includes the molecular weight of either sodium and potassium adducts. This is not an indication that the peptide is not pure, nor should it be confused with an incorrect molecular weight.
Should I cap the N and C termini of my peptide sequence?
AnaSpec recommends that the N-terminal and C-terminal ends of a peptide, mimic the protein of interest. Generally speaking, if the peptide sequence is from the middle of a protein sequence, then both ends of the peptide could be capped to avoid unwanted interactions. AnaSpec recommends that the N-terminal end be capped with an Acetyl group [Ac-NH2] while the C-terminal end be capped with an Amide group [C(O)-NH2].
For peptide sequences that represent the N-terminus, AnaSpec recommends keeping the N-terminus free (NH2) while the C-terminus of the peptide should be amidated [C(O)-NH2].
For peptide sequences that represent the C-terminus, AnaSpec recommends keeping the C-terminus free (COOH) while the N-terminus of the peptide should be acetylated [Ac-NH2].
The above are suggestions only and any modifications or sequence specifications are based on your specific applications.
What methods do you recommend to conjugate a peptide to a carrier molecule?
Maleimide-Thiol Linkage
The preferred method for linking a peptide to the carrier protein is through the side chain thiol (-SH) of a cysteine residue located at either end of the peptide. Usually, the carrier protein is first modified using NBS (m-maleimidobenzoyl-N-hydroxysuccinimide ester). This reagent adds maleimide groups to the surface amines locate on the carrier protein. This maleimide-adduct is then reacted with the thiol group from the peptide and forms a new Sulfur-Carbon bond. The peptide is now conjugated to the surface of the protein via the maleimide linker.
Glutaraldehyde Linkage
Glutaraldehyde cross-links primary amino groups on the peptide to those on the carrier. This linkage is usually employed when there are no available Lys residues present in the peptide sequence. Thus, the N-terminal amine is used as the main point of conjugation.
Carbodiimide Linkage
Carbodiimides couple free carboxyl and amine groups, whether C- or N-terminal or on side chains (i.e lysine, aspartic acid, or glutamic acid) from the peptide to the carrier protein via an amide bond. Amide bonds are extremely rigid and this type of linkage results in considerable steric hindrance as the peptide is tightly bound and close to the carrier protein.
Peptides containing one or more lysine, aspartic acid, or glutamic acid can couple to the carrier protein at various places in the peptide giving alternative conjugates.
MAPS (Multiple Antigenic Peptides)
In some applications (whereby a protein carrier is not necessary) multiple peptide strands coupled to a Lysine backbone may be of great use. Typically 4, 8, or 16 copies of the peptide can be synthesized on a small multivalent core consisting of Lysine. The peptide is synthesized by the usual solid-phase peptide synthesis and cleaved from the resin to give the crude MAP-4, -8, or -16. As the molecular weight of the peptide is now increased several-fold, it may serve as a standalone and not require the linkage to a carrier.
How do I write my peptide sequence termini?
The international nomenclature used for the sequence termini is as follows:
- N-terminus: H means free amine (NH2-), Ac means acetyl [CH3C(O)-NH-], Pyr means pyroglutamic acid
- C-terminus: OH means free acid (-COOH), NH2 means amide [-CONH2]
- Modifications on the side chain of amino acids are depicted in the parenthesis after the corresponding amino acid. For example; phosphorylated serine = S(PO3H2) or epsilon-N-acetylated lysine = K(Ac)
What is a Pyroglutamyl peptide?
When either Glutamine (Q) or Glutamic acid (E) is located at the N-terminus of a peptide sequence, a percentage of the Q or E may spontaneously cyclize to form Pyroglutamate (pGlu). This peptide can now be referred to as a pyroglutamyl peptide. Pyroglutamyl peptides are considered a normal subset of such peptides and are included as part of the peptide purity during HPLC analysis.
pGlu could affect a peptides activity or characteristics and hence have either a positive or negative effect on peptide application. In general, it is believed that the hydrophobic γ-lactam ring of pGlu may play a role in peptide stability against degradation by gastrointestinal proteases.
If only pGlu is desired at the peptide N-terminus AnaSpec also has the capability of replacing the Q or E with pGlu during synthesis. Note that in a peptide sequence, pGlu can also be referred to as Pyr.
What is a peptide counterion?
During peptide manufacturing, ions in the synthesis and purification solutions, bind to the charged amino acid side chains of the peptide, to form salts. These ions are referred to as the peptide counterion and help to balance the peptides electrical charge.
Trifluoroacetic Acid (TFA) is frequently used in peptide synthesis and purification.
It forms a Trifluoroacetate salt with basic amino acids such as Lysine, Arginine, Histidine, and the free N-terminal amine. Hence Trifluoroacetate tends to be the default counterion.
The minimum number of TFA molecules associated with a peptide is directly proportional to the number of basic residues in a given peptide sequence. Therefore, the higher the number of basic residues present in a peptide, the lower the Net Peptide Content of the lyophilized powder. To take into consideration the amount of TFA or water present in the sample, we recommend that the peptide content be determined for every custom peptide.
Water and TFA are byproducts of the purification process. However, TFA can pose toxicity concerns for in-vivo and pre-clinical studies. Therefore, AnaSpec offers non-toxic Acetate, Chloride, or Ammonium salt exchange.
Can AnaSpec conjugate my custom peptide to a carrier protein or antibody?
Yes. As part of our custom peptide synthesis services, we offer conjugation of your peptide to a carrier protein, antibody, or other protein. Common carriers are KLH, cationized BSA, BSA, OVA, and CRM197. With maleimide-thiol linkage being the preferred conjugation method, it is advantageous for the sequence to include a cysteine residue either at the peptide N-terminus or C-terminus.
Can AnaSpec manufacture GLP-1 peptides?
Yes, we manufacture research-grade and GMP-grade GLP-1 peptides.
Our custom GMP GLP-1 peptides, are suitable for pre-clinical studies and clinical development. With this service we also offer stability studies, phase appropriate method validation and complete CMC support.
For research use, we can manufacture your custom sequence or select from one of our off-the-shelf catalog peptides.
See our selection of GLP-1 catalog peptides for research use only.
To learn more about our GMP services and to submit a request please visit: https://www.anaspec.com/en/gmp-peptides
With respect to disulfide bridge cyclization, what does "orthogonal protection" mean?
Orthogonal protection refers to the strategy of using protecting groups on cysteine residues that can be selectively removed under specific conditions—without disturbing other protecting groups on the peptide. This selective removal allows precise control over when and which cysteine residues form disulfide bonds, enabling stepwise and regioselective disulfide bridge formation. Orthogonal protection is especially important when synthesizing peptides with multiple disulfide bonds, as it ensures the desired cysteine pairing and supports proper peptide folding.
Learn more about our disulfide-bridged peptide manufacturing service at: https://www.anaspec.com/en/disulfide-bridged-peptides